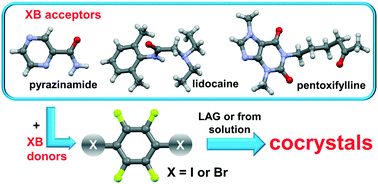
In this work, we present the cocrystallization of three model active pharmaceutical ingredients: pyrazinamide, lidocaine and pentoxifylline. As cocrystal coformers, we selected perfluorinated halogen bond donors, 1,4-diiodotetrafluorobenzene (tfib) and 1,4-dibromotetrafluorobenzene (tfbb). From a crystal engineering standpoint, the compounds are interesting due to the presence of various potential donor and acceptor species.
The choice of structurally equivalent, ditopic linear halogen bond donors, although of different donor strength, allowed us to study the competition between hydrogen and halogen bonding in the obtained solids. We have prepared six cocrystals by both mechanochemical synthesis and a conventional solution-based method. The results of our work show that the dominant supramolecular bond in pyrazinamide cocrystals is the X⋯N type halogen bond (where X = Br or I) established with the pyrazinyl nitrogen atom, in lidocaine cocrystals it is the X⋯O type halogen bond, while in pentoxifylline cocrystals it is dependent on the donor strength. In the tfib cocrystals, halogen bonds are established with both the secondary nitrogen atom and the xanthyl oxygen atom, while in the tfbb cocrystals only Br⋯O halogen bonds are formed.